IRB Committee members
IRB Committee members: Members consist of Fulbright’s scientists, non-scientists, and non-affiliated members (members from the community)
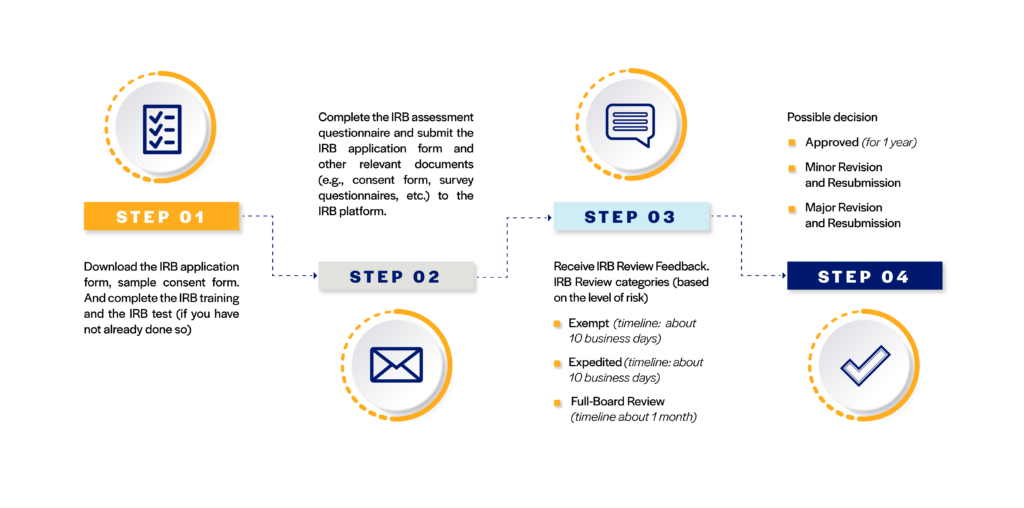
Steps to IRB Application
1. Complete the IRB Training and pass the review test
- IRB Training Requirements: International standards require that key personnel in studies involving human subjects undergo training to ensure research participant safety and welfare. Key personnel are all individuals responsible for the design and conduct of the study. This includes the PI, Co-PI’s, Supervisors and other personnel who will be interacting with the human subject.
- Steps to complete IRB Training: Please watch these IRB training videos to learn about important concepts related to human subject research: IRB Training Video Links
- To take the IRB test, please contact irb@fulbright.edu.vn to add you to the IRB Canvas shell where you can take the test.
- Take the IRB test (100% accuracy required). Multiple attempts are allowed.
- Take a picture of your 100% accuracy results
- est result is valid for 3 years
2. Complete the IRB application documents (application and informed consent documents)
3. Submit the IRB request with all the relevant documents (i.e., application, consent form, all the measures/questionnaires to be used in the study, and the picture of your IRB test results). There will be the IRB risk assessment questionnaire for you to answer when you click on the link before you can attach your documents.
Link to the IRB submission platform:
- IRB submission platform link for UG student
- IRB submission platform link for PG student
- Submit IRB Application for Faculty
Class Assignment and IRB Approval Policy: for all FUV students and instructors, please review these documents.
- Important note: IRB will NOT review applications that are part of student class assignments from any 100-level courses. IRB will also NOT review proposals from student class assignments from most 200-level courses (with only a few exceptions assessed on a case-by-case basis).
- Class Assignment and IRB Policy: Class Assignments & IRB Approval

FAQ

Contact
For more information, please contact us via email at: irb@fulbright.edu.vn
IRB REVIEW PROCESS
IRB approval is required prior to contacting participants or collecting data




